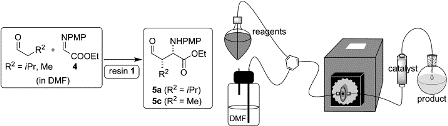
The fast and highly stereoselective Mannich reaction of aldehydes and ketones with the N-(p-methoxyphenyl) ethyl glyoxylate imine catalyzed by polystyrene resins functionalized with (2S,4R)-hydroxyproline is reported. The effect of the nature of the linker connecting proline with the polymeric backbone has been studied, and a 1,2,3-triazole linker constructed from azidomethyl polystyrene and O-propargyl hydroxyproline turns out to be optimal for catalytic activity and enantioselectivity. With aldehyde donors, fast reactions leading to complete conversion in 1-3 h are recorded in DMF. With ketone donors, the reactions tend to be slower, but can be efficiently accelerated (six-membered ring cycloalkanones) by low-power microwave irradiation. This approach, which greatly facilitates product isolation since the catalyst is removed by simple filtration, has allowed the implementation of the reactions of aldehyde substrates in a continuous-flow, single-pass system. In this manner, the continuous synthesis of the enantiomerically and diastereomerically pure adducts (syn/anti>97:3; ee>99 %) has been achieved at room temperature with residence times of 6.0 min. This methodology has allowed for the preparation of up to 7.8 mmol of the desired Mannich adduct through the use of 0.46 mmol of catalytic resin (5.9 mol %), in a greatly simplified experimental protocol that avoids purification steps.