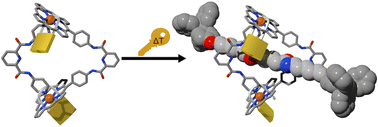
We report the self-assembly of a robust di-nuclear tetralactam macrocyle based on two symmetric components: a Rh(III) bis-porphyrin and a bis-pyridyl ligand. We probe the binding properties of the tetralactam macrocycle with adipamide derivatives using 1H NMR spectroscopy. On the one hand, we show that the binding of the adipamide having linear alkyl chains that can thread through the intact macrocycle’s cavity produces a weakly bound 1 : 1 complex stabilized by four intermolecular hydrogen bonds and featuring a preferred binding geometry of [2]pseudorotaxane topology. On the other hand, we detect the formation of two different complexes in the binding of an analogous adipamide possessing bulky stoppers (dumb-bell axle). The initial addition of the dumb-bell guest induces the formation of a 1 : 1 complex featuring fast exchange kinetics on the 1H chemical shift timescale and exo-cyclic (non-threaded) binding geometry. Notably, in the presence of a large excess of the dumb-bell guest and at suitable concentrations of the macrocycle (>5 mM) we observe the emergence of a second species displaying slow exchange kinetics. This observation allows the undisputed assignment of a [2]rotaxane topology to the second complex. The significant increase in kinetic stability featured by the di-nuclear Rh(III) [2]rotaxane complex contrasts with its reduction in thermodynamic stability (more than one order of magnitude) compared to the previously described di-nuclear Zn(II) counterpart.