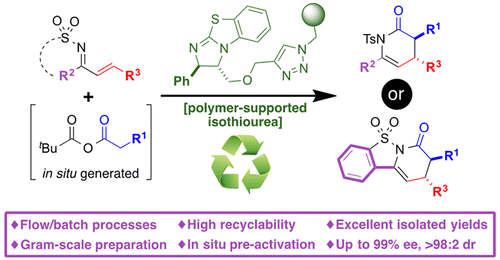
A polystyrene-supported, enantiopure benzotetramisole (BTM) analogue (5) has been synthesized from (2S,3S)-phenylglycidol through a five-step sequence involving a copper-catalyzed alkyne azide cycloaddition (CuAAC) reaction as the final, immobilization step. The functional resin 5 (f = 0.9 mmol g–1) has been successfully used as a highly active and enantioselective catalyst in the domino Michael addition/cyclization reaction of in situ activated arylacetic acids (7) with chalcone-type tosylimines (6), leading to dihydropyridinones 8 (17 examples) to afford the products with excellent yields and very high enantioselectivity (mean ee 97.4%). The deactivation of 5 by species present during the catalytic process has been studied, and pivaloyl chloride (required for the activation of the arylacetic acid) has been identified as the main source of deactivation. A simple experimental protocol taking this fact into account has allowed the multiple recycling of 5 with only a marginal decrease in catalytic activity and the implementation of a continuous flow process where the activation of phenylacetic acid (residence time 14.2 min), the asymmetric domino Michael addition/cyclization reaction (residence time 7.5 min), and aqueous workup are performed sequentially, delivering the dihydropyridinone product as a clean dichloromethane solution (0.54 mmol catalyst sample; 11 h operation; 8a (4.44 g, >99.9% ee)). The supported catalyst 5 has also been used in a new domino Michael addition/cyclization reaction involving saccharin-derived tosylimines 9 as electrophiles, leading to 8,9-dihydro-7H-benzo[4,5]isothiazolo[2,3-a]pyridin-7-one 5,5-dioxides 10 (8 examples) in high isolated yields and diastereoselectivities and excellent enantioselectivities (mean ee 98%). A single sample of 5 (0.5 g, 0.45 mmol) has been used for the sequential preparation in batch of a library of 7 different derivatives 10 at the gram scale (ca. 10 g, accumulated TON = 51), the whole process being performed without any column chromatographic purification. The increased diastereoselectivity recorded with 5 in reactions involving sterically congested arylacetic acids (with respect to homogeneous BTM) has been rationalized through the occurrence of steric interactions between the sulfonylimine and the linker plus support catalyst fragments leading to additional destabilization of the transition state leading to the minor, cis diastereomer of products 8/10.