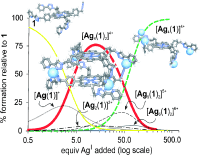
The synthesis of a zinc(II) porphyrin 1 with four appended triazolyl–pyridine chelates is reported. Complexation of the porphyrin peripheral ligands with AgI ions in a 1:2 binding stoichiometry afforded quantitatively the coordination cage [Ag4(1)2]4+. The assembly and disassembly processes of the cage were investigated in solution using UV/Vis spectroscopy. The mathematical analysis of the data obtained in the UV/Vis titration of 1 with AgI confirmed the assembly in CH2Cl2/MeOH (90:10) solution of a species having a 1:2 porphyrin/silver stoichiometry and assigned to it an overall stability constant of 5.0×1026 M−5. The use of a model system allowed an independent assessment of a microscopic binding constant value (Km) for the interaction between the triazolyl-pyridine ligand and AgI. The coincidence that existed between the Km values extracted from the model system and the titration of 1 provided an indication of the quality and fit of the data analysis. It also allowed the calculation of the average effective molarity (EM) value for the three intramolecular processes that led to the cage assembly as 2.6 mM. Simulated speciation profiles supported the conclusion that at millimolar concentration and working under strict stoichiometric control of the silver/porphyrin ratio, the cage [Ag4(1)2]4+ was the species exclusively assembled in solution. On the other hand, when the concentration of added AgI was approximately 2.6 mM, 50 % of the coordination cage disassembled into open aggregates.