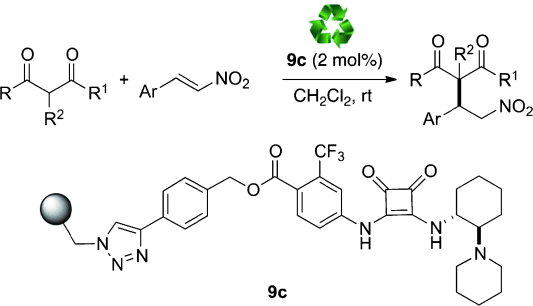
A chiral squaramide has been supported onto a polystyrene (PS) resin through a copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction and used as a very active, easily recoverable and highly reusable organocatalyst for the asymmetric Michael addition of 1,3-dicarbonyl compounds to β-nitrostyrenes. The PS-supported squaramide could be recycled up to 10 times.