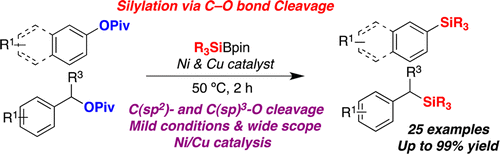
A Ni/Cu-catalyzed silylation of unactivated C–O electrophiles derived from phenols or benzyl alcohols is described. This transformation is characterized by its wide scope and mild conditions, providing a direct access to synthetically versatile silylated compounds. The protocol allows for the coupling of C(sp2)−O and even C(sp3)–O bonds with similar efficiency.