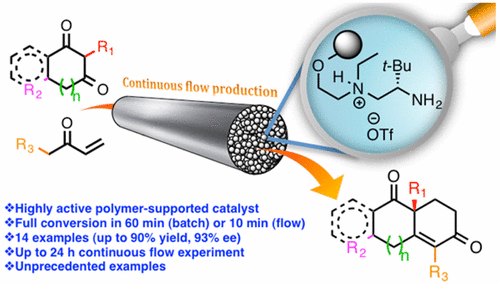
The preparation through Robinson annulation of enantiopure building blocks with both academic and industrialrelevance, such as the Wieland-Miescher and Hajos-Parrish ketones, has suffered from important drawbacks, such as the need of high catalyst loading or extremely long reaction times. Here we report a heterogeneized organocatalyst based on Luo’s diamine for the fast and broad-scope enantioselective Robinson annulation reaction. The polystyrene-supported diamine 19a enables the high-yield, highly enantioselective preparation of a wide scope of chiral bicyclic enones under mild conditions, with reactiontimes as short as 60 minutes (batch) or residence times of 10 minutes (flow). In contrast with its homogeneous counterpart 19b, the catalytic resin 19a experiences a notable increase in catalytic activity with temperature in 2-MeTHF (a ten-fold decrease in reaction times without erosion in enantioselectivity is observed from room temperature to 55 ºC). The scope of the transformation in batch has been illustrated with 14 examples, including examples only reported in poorly enantioenriched (22n) or in racemic form (22k). Enantiopure 22k has been used asstarting material for a straightforward formal synthesis of the antibiotic and antifeedant sesquiterpene (-)-isovelleral (24). The heterogeneized catalyst 19a admits extended recycling (10 cycles) and has been used to develop the first asymmetric Robinson annulations in continuous flow. The potential of the flow process is illustrated by the large scale preparation of the Wieland-Miescher ketone (65 mmol in 24 h operation, TON of 117) and by a sequential flow experiment leading to a library of eight enantioenriched diketone compounds.