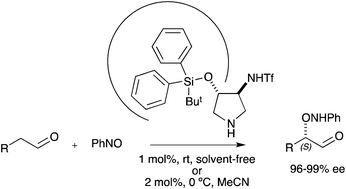
Enantiopure trans-3-trifluoromethylsulfonylamino-4-silyloxypyrrolidines efficiently catalyse the asymmetric α-aminoxylation of aldehydes. At 1% catalyst loading (solvent-free conditions) or at 2% catalyst loading (acetonitrile solution) aldehydes are completely converted in short reaction times leading to α-aminoxylation products with very high (96-99%) enantioselectivity.