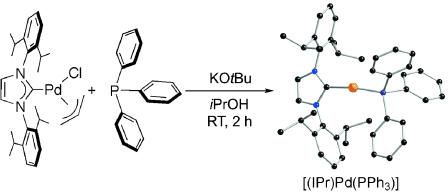
Mixed NHC-phosphane palladium(0) complexes [(NHC)Pd(PR3)] (NHC: N-heterocyclic carbene) are synthesized directly from commercially available reagents, with the possibility to tune the nature of both the NHC and the phosphane. Reaction of [(NHC)Pd(allyl)Cl] (palladium source) and PR3, in the presence of a base afforded, in isopropanol, [(NHC)Pd(PR3)] in good yields. We found that the nature of the solvent played a key role in the efficient reduction of the PdII precursor to Pd0. Supported by experimental evidence we propose that the reduction step is driven by the isopropoxide anion formed in situ from isopropanol and a base. Detection of acetone in the reaction mixture confirms that the isopropoxide anion acts as the reducing agent. Moreover, different bases proved efficient for the reaction. The structures of the complexes were unambiguously confirmed by X-ray analysis. Exposure of these complexes to air does not lead to decomposition, but to the oxo-complex [(NHC)Pd(PR3)(O2)], which is stable both in the solid state and in solution.