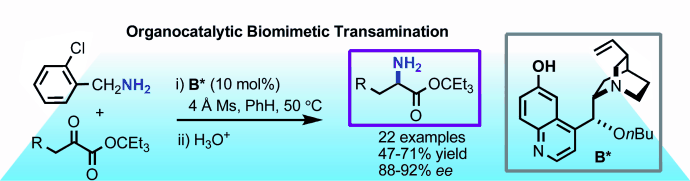
The instinctive desire of organic chemists to imitate the catalytic machinery of enzymes has brought about the development of the first catalytic highly enantioselective synthesis of α-amino acid derivatives by biomimetic transamination. Key to success was the ability of a cinchona-based chiral catalyst to mimic the general base-acid catalytic mechanism of the natural transaminases.