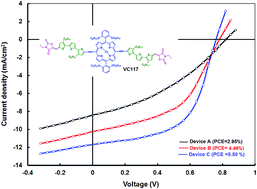
In this article, we have designed and synthesized a porphyrin with the following molecular architecture A–π–D–π–A in which ethyl rhodanine end capping groups were linked to the core porphyrin donor via an octyl thiophene-ethynylene π bridge denoted as VC117 and used it as an electron donor along with ([6,6]-phenyl C71 butyric acid methyl ester) (PC71BM) as an electron acceptor for the fabrication of solution processed organic solar cells. The solution processed BHJ organic solar cell with an optimized weight ratio of 1 :
: 1 VC117
1 VC117 :
: PC71BM in THF (tetrahydrofuran) showed an overall power conversion efficiency (PCE) of 2.95% with short circuit current Jsc = 8.34 mA cm−2, open circuit voltage Voc = 0.82 V and fill factor FF = 0.43. Nonetheless, when the active layer of the solar cell was processed from a mixture of 4% v/v of pyridine in THF solvent, it achieved a PCE value of 4.46% and further improved up to 5.50% after thermal annealing. This is ascribed to the enhancement of both the Jsc and FF values. The higher value of Jsc is explained by the increased absorption profile of the blend, the higher incident photon to current efficiency (IPCE) response and the better crystallinity of the active layer when processed with solvent additives and thermal annealing while the enhancement of FF is due to the better charge transport capability and the charge collection efficiency of the latter device.
PC71BM in THF (tetrahydrofuran) showed an overall power conversion efficiency (PCE) of 2.95% with short circuit current Jsc = 8.34 mA cm−2, open circuit voltage Voc = 0.82 V and fill factor FF = 0.43. Nonetheless, when the active layer of the solar cell was processed from a mixture of 4% v/v of pyridine in THF solvent, it achieved a PCE value of 4.46% and further improved up to 5.50% after thermal annealing. This is ascribed to the enhancement of both the Jsc and FF values. The higher value of Jsc is explained by the increased absorption profile of the blend, the higher incident photon to current efficiency (IPCE) response and the better crystallinity of the active layer when processed with solvent additives and thermal annealing while the enhancement of FF is due to the better charge transport capability and the charge collection efficiency of the latter device.