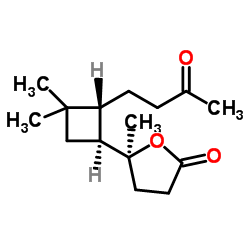
The new strategy, developed by Prof. Echavarren’s group, was used to produce a wide scope of more than 40 products, including anti-tumour agent rumphellaone A.
Prof. Echavarren’s group presents the first general enantioselective synthesis of cyclobutenes by intermolecular [2+2] cycloadditions. Digold complexes catalyse the reaction between terminal alkynes and alkenes leading to chiral cyclobutenes, useful in organic chemistry because they can be hydrogenated into functionalised cyclobutanes, which are motifs present in a variety of natural products and pharmaceuticals.
Previous attempts to achieve this transformation required activated alkynes or strained alkenes. The new strategy developed at ICIQ works for an enormous variety of terminal alkynes and a myriad of both disubstituted and trisubstituted alkenes. In total, this gold-catalysed reaction was used to synthesize a scope of over 40 different products, all with good yields and excellent enantiomeric ratios.
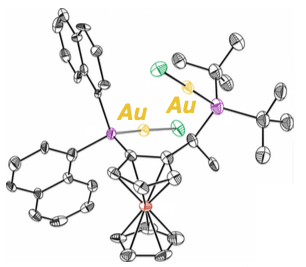
Due to the linear geometry of gold(I) complexes, chiral ligands are usually far away from the reaction centre. Hence, finding the gold catalysts that yield enantioselective transformations is a hard process. To do this, Echavarren’s team used CELLEX-ICIQ high throughput facilities to test up to 90 different chiral ligands. A family of Josiphos ligands, in particular the digold complex pictured below, gave the best results in terms of yield and enantioselectivity.
Additionally, researchers demonstrated that having a digold complex –with two gold atoms– is key to achieve these unprecedented results. After carrying out experimental and computational studies, they demonstrated one of the gold(I) centres activates the alkyne, while the other is directly involved in the induction of enantioselectivity.
As a proof of concept of the advantages of this transformation, Echavarren’s team used it to prepare anti-tumour agent rumphellaone A in only 9 steps, improving their own previously reported total synthesis.
Reference:
Enantioselective Synthesis of Cyclobutenes by Intermolecular [2+2] Cycloaddition with Non-C2 Symmetric Digold Catalysts
C. García-Morales, B. Ranieri, I. Escofet, L. López-Suárez, C. Obradors, A.I. Konovalov, A.M. Echavarren
J. Am. Chem. Soc. 2017, 139, 13628-13631, DOI: 10.1021/jacs.7b07651.