CC-BY-NC The Royal Society of Chemistry 2018
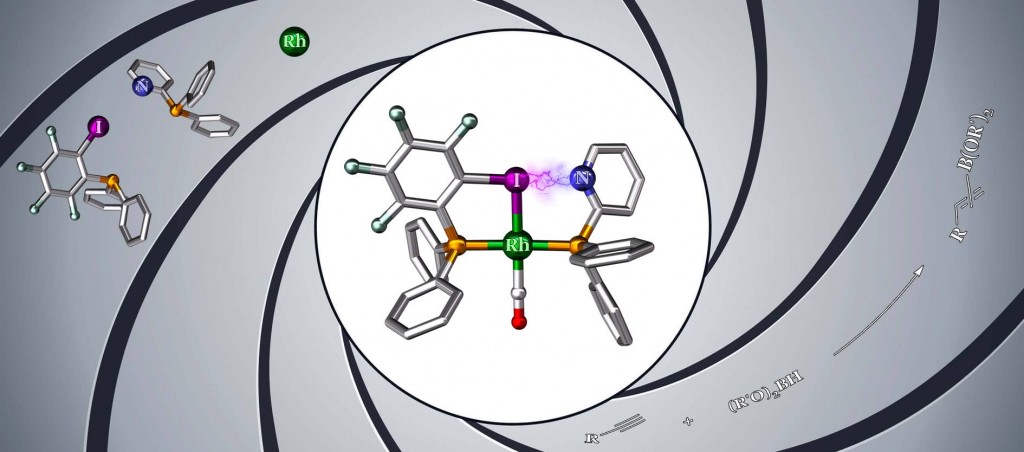
The XBphos ligand constitutes the first example of halogen bonding
as driving force for the assembly of supramolecular catalysts.
Supramolecular chemistry is nowadays considered an efficient tool for the construction of ligand backbones in catalytic systems. Chemists have been successfully using this approach for preparing catalysts by assembling supramolecularly complementary building blocks through many types of supramolecular interactions, but halogen bonding remained unexplored.
Lucas Carreras and Prof. Anton Vidal, in collaboration with the group of Prof. Piet van Leeuwen (currently at INSA, Toulouse), developed a new strategy to construct a bisphosphine catalyst backbone that self assembles around a rhodium atom. This is possible thanks to the formation of a halogen bond between pyridyl- and iodo-substituted monophosphines. The structure of this unique supramolecular complex was confirmed by X-ray crystallography, amongst other spectroscopic techniques.
Researchers also carried out computational studies, which, among other findings, revealed that the formation of the halogen bond was energetically favourable. Moreover, DFT calculations corroborated the existence of a favourable σ-hole in the iodo-substituted monophosphine, characteristic of this type of non-covalent bonding.
As a proof of concept, the authors evaluated the reactivity of these new catalysts in the hydroboration of terminal alkynes. The halogen bonded bisphosphine outperforms known monodentate and bidentate ligands in terms of yield. It also shows an interesting selectivity, favouring in a number of examples the highest ever reported ratios of the branched alkenyl boronic acid derivatives.
XBphos-Rh: A Halogen-Bond Assembled Supramolecular Catalyst
L. Carreras, M. Serrano-Torné, P.W.N.M. van Leeuwen, A. Vidal-Ferran
Chem. Sci. 2018, DOI: 10.1039/C8SC00233A.
* Highlighted in Chemistry Views.