Abstract
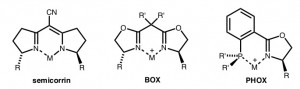
For a long time, C2-symmetric ligands were dominating in asymmetric catalysis. In our research in asymmetric catalysis, the first ligands we designed were also C2-symmetric. However, subsequently an increasing number of reactions was found, in which nonsymmetric bidentate P,N-ligands outperformed C2-symmetric P,P- or N,N-ligands. Especially phosphino-oxazolines (PHOX ligands) have emerged as highly effective widely applicable ligands. In this lecture the concepts will be discussed that led us from semicorrins and bisoxazoline (BOX) ligands to the PHOX ligands.
As a major application of P,N-ligands, the asymmetric Ir-catalyzed hydrogenation of olefins will be discussed. In contrast to rhodium- and ruthenium-phosphine complexes, these catalysts do not require the presence of a coordinating group next to the C=C bond and, therefore, have considerably extended the scope of asymmetric hydrogenation.
In the second part of the lecture, a new screening method for chiral catalysts will be described that is based on mass-labeled quasi-enantiomeric substrates and electrospray ionization mass spectrometry as an analytical tool. This method allows determination of the intrinsic enantioselectivity of a catalyst by mass spectrometric monitoring of catalytic intermediates. In contrast to conventional screening methods, which are based on product analysis, simultaneous screening of catalyst mixtures in homogeneous solution is possible. In addition, mechanistic insights about the enantioselectivity-determining step of a catalytic cycle may be gained.