Abstract
Recycling of CO2 is regarded as a technology necessary for next generation beyond solar hydrogen production by water splitting reaction, because organic chemicals are considered to be more useful than hydrogen if they were synthesized from CO2. In this presentation, I will introduce our research results on the solar CO2 reduction in an aqueous solution conducted around ambient temperature and normal pressure.
We have proposed the novel concept of selective CO2 photoreduction using a hybrid photocatalyst comprising of p-type N-doped Ta2O5 semiconductor photosensitizer linked with a Ru-complex electrocatalyst ([Ru(dcbpy)2(CO)2]2+), in which conduction band electrons in photoexcited N-Ta2O5 transferred to the Ru-complex within tens of picoseconds. Then we have proven that a photocahode of zinc-doped indium phosphide (InP) coated with a ruthenium complex polymer ([Ru{4,4’-di(1H-pyrrolyl-3-propylcarbonate)2,2’-bipyridine}(CO)2]n, RuCP) electrocatalyst can reduce CO2 to formate with high selectively (>70%) under visible light irradiation in water. By wiring the InP/[RuCP] photocathode for CO2 reduction with a TiO2 or SrTiO3-x photoanode for H2O oxidation in two compartment cell separated with proton exchange membrane, solar formate production by the Z-scheme reaction utilizing only sunlight, CO2 and H2O was successfully realized at solar-to-chemical conversion efficiencies (SCE) of 0.04-0.14 % without an external electrical bias application. Development of new metal-complex catalysts was also conducted, and the semiconductor/metal-complex system has recently been integrated to a monolithic tablet-shaped device of [RuCP]/carbon/SiGe-jn/IrOx. By applying functions of selective reduction /oxidation reactions to both catalytic sites, the device operated in a single-compartment reactor in phosphate buffer at pH =6.4 (Fig.1), and it generated formate with a very high SCE of 4.6 %.
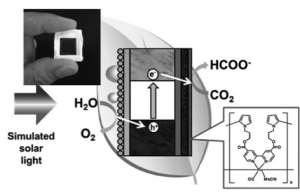
Fig. 1 Schematic illustration of a monolithic tablet-shaped device for CO2 photoreduction in an aqueous solution.
