Abstract
Radicals are intrinsically reactive, and were long believed to be “too reactive to be selective”. However, in the coordination sphere of transition metals highly selective radical-type processes are certainly possible. In fact, radical-type reactions are tremendously important in several bio-synthetic pathways mediated by metallo-enzymes. Nature solves its most difficult and most interesting bio-synthetic problems with radical-reactivity. Yet, despite their radical-nature, these reactions proceed with ultrahigh precision and selectivity.
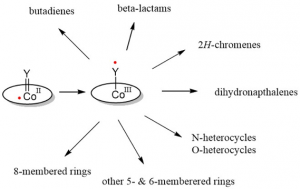
Inspired by such intriguing catalytic radical-type transformations mediated by metallo-enzymes, we are investigating new catalytic radical-type transformations mediated by synthetic (open-shell) organometallic catalysts. This presentation is focused on the diverse radical-type reactivity of cobalt-carbene (and nitrene) complexes, in which the transient reactive moieties act as redox active ligands producing discrete carbene (and nitrene) radicals. Such species provide unique opportunities in developing new catalytic ring-closure protocols. Here we report on their diverse radical-type pathways, revealing both convergent pathways and unique divergent routes to a variety of desirable organic ring products.