Abstract
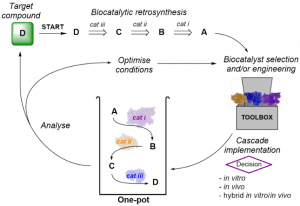
This lecture will describe recent work from our laboratory aimed at developing new biocatalysts for enantioselective organic synthesis, with emphasis on the design of in vitro and in vivo cascade processes for generating chiral pharmaceutical building blocks. By applying the principles of ‘biocatalytic retrosynthesis’ we have shown that it is increasingly possible to design new synthetic routes to target molecules in which biocatalysts are used in the key bond forming steps. The integration of several biocatalytic transformations into multi-enzyme cascade systems, both in vitro and in vivo, will be addressed in the lecture. In this context monoamine oxidase (MAO-N) has been used in combination with other biocatalysts and chemocatalysts in order to complete a cascade of enzymatic reactions. Other engineered biocatalysts that can be used in the context of cascade reactions include transaminases, ammonia lyases, amine dehydrogenases, imine reductases, and artificial transfer hydrogenases. We shall also present recent work regarding the discovery of a new biocatalyst for enantioselective reductive amination and show how these enzymes can be used to carry out redox neutral amination of alcohols via ‘hydrogen borrowing’.