Abstract
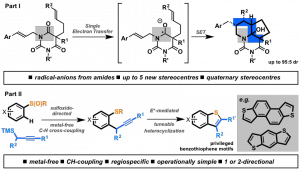
The first part of the lecture will describe the development of radical-radical cyclization cascades, triggered by electron-transfer reduction of amides using SmI2,1 for the generation of complex heterocyclic systems.2,3 The second section will discuss the development of sulfoxide-directed4 metal-free CH cross-coupling processes,5 including CH–CH-type couplings,6 and their application in the synthesis and late-stage manipulation of bioactive targets and organic materials.5-7
References
- (a) Just-Baringo, X.; Procter, D. J. Acc. Chem. Res. 2015, 48, 1263. (b) Szostak, M.; Fazakerley, N. J.; Parmar, D.; Procter, D. J. Chem. Rev. 2014, 114, 5959
- Huang, H-M.; Procter, D. J. J. Am. Chem. Soc. 2016, 138, 7770.
- Huang, H-M.; Procter, D. J. Unpublished results, 2016.
- Pulis, A. P.; Procter, D. J. Angew. Chem. Int. Ed. 2016, 55, 9842.
- (a) Eberhardt, A. J.; Procter, D. J. Angew. Chem. Int. Ed. 2013, 52, 4008. (b) Eberhart, A. J.; Shrives, H. J.; Álvarez, E.; Carrër, A.; Zhang, Y.; Procter, D. J. Chem. -Eur. J. 2015, 21, 7428. (c) Eberhart, A. J.; Shrives, H.; Zhang, Y.; Carrër, A.; Parry, A. V. S.; Tate, D. J.; Turner, M. L.; Procter, D. J. Chem. Sci. 2016, 7, 1281.
- Fernández-Salas, J. A.; Eberhart, A. J.; Procter, D. J. J. Am. Chem. Soc. 2016, 138, 790.
- Fernández-Salas, J. A.; Shrives, H. J.; Pulis, A. P.; Procter D. J. Chem. Commun. 2016, 52, 12364.
Seminar funded by: