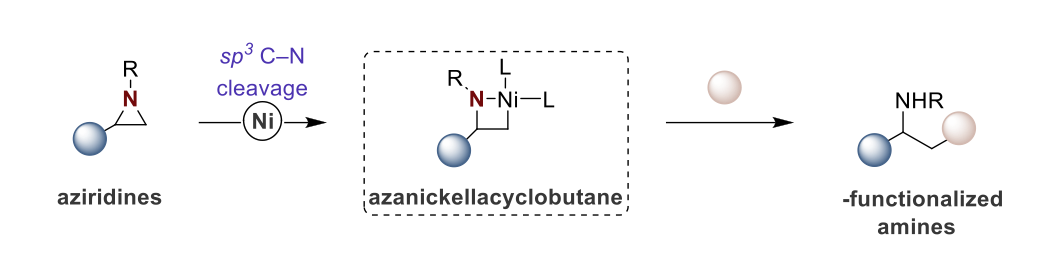
Beta-functionalized amines hold importance in the chemical industry, with a growing demand for novel synthetic approaches. Over the last decade, there has been considerable interest in transition metal-catalyzed cross-coupling reactions involving aziridines as substrates to produce beta-functionalized amines. Early studies by Hillhouse and Wolfe, who identified key reaction intermediates known as azametallacyclobutanes, paved the way for subsequent elegant protocols in this field. We identified the potential of accessing beta-amino acids and homoallylamines from aziridines by coupling them with carbon dioxide or simple alkynes. Through systematic optimization of reaction conditions, we successfully developed Ni-catalyzed reductive carboxylation and hydroalkenylation methodologies, broadening their synthetic applicability across various starting materials while identifying certain reaction limitations. Mechanistic investigations provided insights into the underlying processes, leading to the design of a new Ni-catalyzed electrophile cross-coupling transformation. In summary, this doctoral thesis offers a new protocol for leveraging the potential of aziridines as valuable building blocks to synthesize added-value chemicals, particularly beta-amino acids and homoallylamines.