Abstract
Halogen bonding denotes the noncovalent interaction between electrophilic halogen substituents and Lewis bases. Although it shares many similarities with hydrogen bonding, it has received little interest until the last two decades. Even less explored is chalcogen bonding, which is based on electrophilic atoms of the 6th main group.
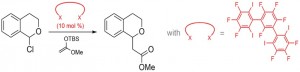
The main goal of our research is to develop applications of halogen bonding in solution, with a strong focus on multidentate halogen bond donors and their use in organocatalysis. By now, we have successfully employed these compounds as activators or catalysts in test reactions for halide abstraction or neutral molecule activation (see Figure 1).
Figure 1: Halogen-bond-catalyzed halide abstraction reaction.
Lately, we have also extended this concept towards hypervalent iodine(III) derivatives as Lewis acids. In parallel, we are also interested in multipoint halogen bonding, i.e. the interaction of several halogen substituents on one molecule with several Lewis basic sites on a second molecule. As an example, we introduced the three-point halogen-bonded complex between a polyfluorinated and -iodinated quaterphenyl and an orthoamide.
Finally, we aim to establish chalcogen bonding as a further tool in organocatalysis. Bidentate organoselenium derivatives with cationic backbones were used as activators or catalysts in benchmark reactions for carbon-halogen bond activation, and it could be shown that the observed activity is very likely based on chalcogen bonding.